Integrated, GMP-aligned Manufacturing
Capabilities
Are you looking for established and proven med-tech engineering, manufacturing, and quality expertise?
You are in the right place.
Engineering, Quality,
Validation and Supply chain
Quvara Medical provides integrated, GMP-aligned manufacturing and technical services from its Swindon, UK med-tech hub, supporting pharmaceutical and biotechnology customers from early industrialisation through to sustained, high-volume commercial supply.
With over 30 years of regulated manufacturing heritage, Quvara operates as a high-performance CMO purpose-built for regulated scale. Operations run 24/7 across four shifts, supported by 350 associates and deeply integrated quality, validation, engineering, and digital systems.

Our Capabilities
Contract Manufacturing
Quvara provides GMP-compliant contract manufacturing for pharma and biotech customers requiring absolute confidence in product integrity, process control, and continuity of supply.
Manufacturing operations run continuously, supported by automated and semi-automated assembly lines, over 50 injection moulding machines, ISO Class 7 & 8 cleanrooms, and in-line inspection. On-site prototyping and test method development enable rapid iteration and robust process control.
This delivers predictable scale-up, reduced lifecycle risk, and confidence that manufacturing will not constrain commercial success.

Device Platform Support
Quvara supports device and delivery platforms rather than isolated products, enabling customers to manage pipelines, variants, and geographic expansion without repeated reinvention.
Platform support includes controlled configuration management, reusable tooling strategies, and validation approaches designed to support evolution while maintaining regulatory integrity.

Engineering & Industrialisation
Quvara’s engineering teams ensure products transition efficiently from design intent to validated commercial manufacture.
Capabilities include DFM, DFA, process development, automation strategy, tooling development, line commissioning, and industrial transfer. Advanced tools such as 3D printing and CT scanning enable faster risk identification and root-cause analysis.

Quality & Validation Services
Quality and validation are foundational capabilities at Quvara.
We provide full lifecycle validation services including IQ, OQ, PQ, equipment qualification, Risk Management as part of FMEA, electronic batch records, automated release, and real-time quality trending. Systems are fully compliant with ISO 13485 and FDA 21 CFR Part 820, ensuring continuous inspection readiness.

Supply Chain & Procurement
Quvara manages complex, regulated supply chains with a focus on resilience, transparency, and compliance.
Capabilities include supplier qualification, dual-sourcing strategies, capacity planning, and full material traceability — reducing exposure to shortages, disruptions, and compliance risk.

Packaging & Labelling
Packaging and labelling are fully integrated into Quvara’s regulated manufacturing workflows.
Capabilities include cleanroom packaging, label verification, serialisation-ready workflows, and integration with validated quality systems, supporting efficient global market release.

Data & Digital Integration
Quvara operates digitally enabled manufacturing as standard.
Electronic batch records, automated quality release, and integrated data systems support traceability, real-time insight, and audit-ready documentation across the product lifecycle.
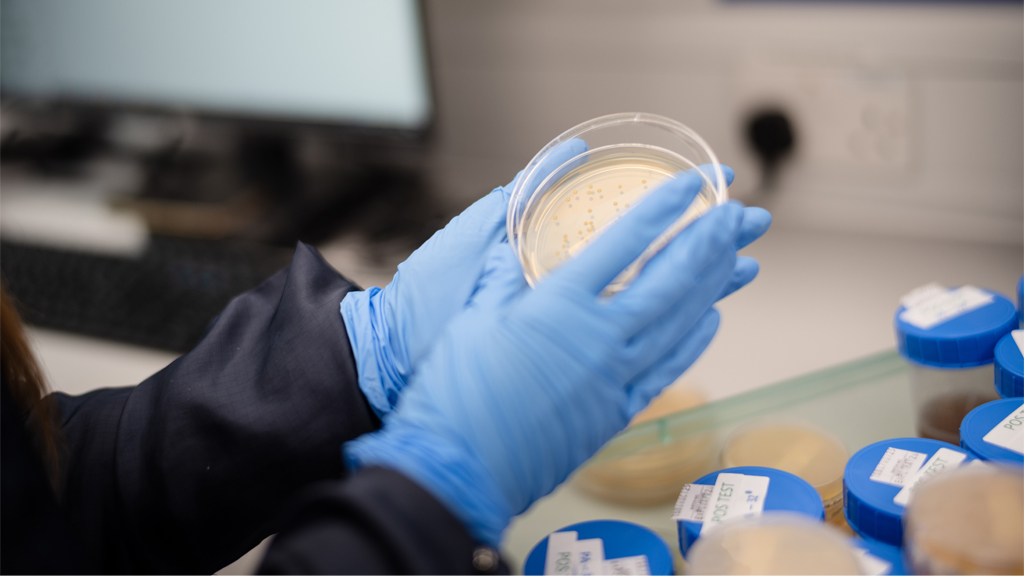
Microbiology Testing
Quvara operates a fully integrated, in-house microbiology function aligned with EU GMP and global regulatory expectations. Our capability supports routine environmental monitoring, cleanroom performance trending, and timely investigation of microbiological deviations, forming a core part of our contamination control strategy.
By embedding microbiology expertise directly within manufacturing operations, we maintain validated states, support batch release decisions, and enable rapid, evidence-based responses to emerging risks. This integrated approach provides regulators and customers with confidence in product quality, process control, and long-term supply reliability.

Precision. Compliance. Trust.
Struggling to find the answer you are looking for?
We have a team of experts ready to help with your queries.